AbstractPurposeThis study investigated the effects of healthy lifestyle interventions (HLSIs) on health-related quality of life (HR-QoL) in childhood and adolescent cancer survivors (CACS).
MethodsMajor databases were searched for English-language original articles published between January 1, 2000 and May 2, 2021. Randomized controlled trials (RCTs) and non-RCTs were included. Quality was assessed using the revised Cochrane risk-of-bias tool, and a meta-analysis was conducted using RevMan 5.3 software.
ResultsNineteen studies were included. Significant effects on HR-QoL were found for interventions using a multi-modal approach (exercise and education) (d=-0.46; 95% confidence interval [CI]=-0.84 to -0.07, p=.02), lasting not less than 6 months (d=-0.72; 95% CI=-1.15 to -0.29, p=.0010), and using a group approach (d=-0.46; 95% CI=-0.85 to -0.06, p=.02). Self-efficacy showed significant effects when HLSIs provided health education only (d=-0.55; 95% CI=-0.92 to -0.18; p=.003), lasted for less than 6 months (d=-0.40; 95% CI=-0.69 to -0.11, p=.006), and were conducted individually (d=-0.55; 95% CI=-0.92 to -0.18, p=.003). The physical outcomes (physical activity, fatigue, exercise capacity-VO2, exercise capacity-upper body, body mass index) revealed no statistical significance.
INTRODUCTIONOver the past 40 years, the incidence of childhood and adolescent cancer has been steadily increasing worldwide [1]. Although advances in treatments and supportive care have led to a 5-year survival rate of over 80%, particularly in developed countries, the number of childhood and adolescent cancer survivors (CACS) is expected to increase further in the coming decades [2].
With improved survival, CACS are unfortunately at risk for a myriad of late effects [3]. Late effects occur because the treatment needed to cure the primary cancer can lead to chronic health problems in survivors [4]. After treatment completion, CACS face a high risk of both physical and psychological problems, such as endocrine dysfunction, cardiovascular disease, chronic fatigue, stress, anxiety, and depression, as well as subsequent malignant neoplasms [4-6]. These comorbid conditions can worsen the patient's emotional health and psychosocial adjustment and are associated with lower health-related quality of life (HR-QoL) among CACS [5-7]. Several previous systematic reviews and meta-analyses have reported that these comorbidities are often progressive and strongly influenced by a healthy lifestyle, including physical activity, adopting a healthy diet, not smoking, limiting alcohol intake, and psychosocial adjustment [5,6,8-13]. Therefore, effective lifestyle interventions to prevent the trajectory of comorbidities and strategies to promote HR-QoL are important healthcare issues that cannot be overlooked.
The World Health Organization (WHO) defines lifestyle as ‘‘identifiable behavioral patterns, determined by the interaction between individual personal characteristics, social interactions, and socioeconomic and environmental life conditions'' [14]. Healthy behaviors established during adolescence help determine one's health status later in life and affect the risk of developing chronic diseases [15]. A healthy lifestyle can reduce the risk of preventable health problems and improve one's HR-QoL [16]. A healthy lifestyle can be defined as patterns of behavior that help to maintain or improve people's health and well-being. The association between healthy lifestyle factors and HR-QoL is cumulative, underscoring the importance of promoting multiple healthy lifestyles to enhance HR-QoL in long-term survivors of childhood cancer [16,17]. Furthermore, previous studies have shown relationships between (lack of) exercise, HR-QoL, and fatigue, and there is a need to improve self-efficacy by connecting CACS with relevant educational resources [18,19]. However, in the last 5 years, although several systematic reviews and metaanalyses related to health outcomes or interventions to improve the health of CACS have been published, they have only focused on health behaviors such as exercise or diet management [5,6,8-11,20] or have investigated the effects of interventions related to emotional coping [12,13].
The concept of a healthy lifestyle does not simply refer to behavior, but includes multiple aspects of one's overall lifestyle [16, 21]. To improve the HR-QoL of CACS, it is necessary to identify the effect size of comprehensive interventions that promote a healthy life with various intervention modalities such as internet/online programs, face-to-face interventions, and educational games. This study aimed to comprehensively identify the effects of interventions targeting CACS on each factor affecting a healthy lifestyle.
METHODS
Ethics statement: The Institutional Review Board (IRB) of Sahmyook University (No. 2020133HR) reviewed this study. The committee decided that this study was an exempt research study.
1. Study DesignThe systematic review and meta-analysis conducted in this study conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The protocol for this review has been registered in the International Prospective Register for Systematic Reviews (PROSPERO; registration ID: CRD42021227840).
2. Eligibility CriteriaThe authors followed the participants, interventions, controls, outcomes, and studies (PICOS) framework. The target population for our review was child and adolescent survivors (after completion of their cancer treatment) with childhoodonset cancer (<18 years of age), regardless of the type of cancer and duration of survivorship. Studies that included patients who were undergoing active therapy, or with insufficient data (e.g., mean, standard deviation, and p-values) to calculate the effect size were excluded from our analysis.
We included randomized controlled trials (RCTs) and non-RCTs with a comparison group regarding healthy lifestyle interventions (HLSIs). To identify intervention effects regarding the HR-QoL of CACS, a more comprehensive approach to healthy lifestyle improvement strategies based on the WHO's health definition was required. The HLSIs in this review included healthy physical behaviors (e.g., exercise, good nutrition, and avoidance of alcohol and smoking) and psychosocial and spiritual interventions to enhance positive adherence and the HR-QoL of CACS. The modalities included face-to-face interventions, electronic health (eHealth) interventions, and mobile health (mHealth) interventions.
3. Search StrategySeveral databases were searched to locate relevant studies: MEDLINE via PubMed, Embase, PsycINFO, Cochrane Library, CINAHL, and multiple Korean web-based databases (KCI, KISS, KMBASE, KoreaMed, NDSL, RISS, and ScienceON). The search included English-language original articles published between January 1, 2000 and May 2, 2021. The major search terms used were as follows: ("Pediatrics" OR "Adolescent" OR "Child" OR "Childhood") AND ("Neoplasms" OR "Hematologic Neoplasms" OR "Leukemia" OR "Lymphoma" OR "Hodgkin Disease") AND ("Survivors" OR "Cancer Survivors") AND ("Healthy Lifestyle" OR "Health Behavior" OR "Psychosocial Intervention" OR "Internet-Based Intervention" OR "Spiritual Therapies" OR "Psychotherapy" OR "Exercise" OR "Diet Therapy" OR "Health Promotion") AND ("Quality of Life" OR "Self-Efficacy" OR "Adaptation" OR "Psychosocial Functioning" OR "Self-Management" OR "Posttraumatic Growth, OR "Academic Performance").
The electronic search was supplemented with a manual review of reference lists from relevant publications to locate additional publications. The "gray" literature (e.g., abstracts, conference proceedings, editorials, and dissertations) and reviews were excluded.
4. Study Selection and Data ExtractionThe data extraction process employed the PRISMA procedure. After the search terms and strategies were finalized, the data bases were searched by four authors (K.K.A., H.Y.K., Y.L.O., and H.J.Y.). Two authors (K.K.A. and H.Y.K.) independently extracted all titles and abstracts, removed duplicates, and screened them for relevance and suitability according to the inclusion criteria. After screening, a full-text review was conducted to select final articles for inclusion. Disagreements were resolved through discussion with two other authors (S.J.H. and J.Y.C.) and the reasons for excluding studies were documented. The extracted articles were organized as follows: study authors, publication year, country of study, study design, sample size, intervention (name, type, providers, duration, session number, length per session, format [e.g., individual, group, or mixed]), outcome variables, and measurements. If study outcomes were reported more than once, the studies in which the effects of specific interventions were confirmed with varying study periods and subjects over repeated studies were included.
5. Quality Assessment of the Studies IncludedThe quality of each individual study was assessed independently by two authors (K.K.A. and S.J.H.) and verified by a second reviewer (J.Y.C.). RCTs were assessed using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (revised on August 22, 2019, by the RoB 2 Development Group) [23]. The five domains of RoB 2 were the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Respondents selected "yes," "probably yes," "probably no," "no," or "no information" for each item. In addition, the purpose of this meta-analysis was to confirm the difference in effect between the experimental group and the control group. Therefore, the quality of selected papers was evaluated based on the intention-to-treat (ITT) method of effect confirmation. Non-RCT studies were reviewed using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [24]. The risk of bias was evaluated and classified as low, moderate, serious, or critical for seven domains (confounding; selection of participants into the study; classification of intervention; deviations from intended interventions; missing data; measurement of outcomes; and selection of the reported results) within each study. The overall risk of bias was judged for each study and classified as low, moderate, serious, or critical using the least favorable assessment of the seven domains.
6. Statistical AnalysisWe performed a meta-analysis using the Cochrane Collaboration's Review Manager (RevMan 5.3) [25]. A randomeffects model was used to compute the mean difference between pre- and post-intervention data. The level of significance was set at p<.05, and all statistical tests were two-sided. The effect size was calculated as the standardized mean difference (SMD) with a 95% confidence interval (CI). Outcome means and standard deviations were used to compute the SMD (Cohen's d).
A Cohen's d of 0.8 was considered large, 0.5 medium, and 0.2 small [26]. Heterogeneity (apparent diversity in the weighted mean differences across studies) was examined using the x2 distribution using the I2 statistic [23]. In addition, a subgroup analysis was performed in two groups according to intervention type, period, and format (individual or group). Publication bias was screened using funnel plots, and if the results were evenly distributed within the triangular shape, it was interpreted that publication bias had not occurred [27].
RESULTS1. Study SelectionAccording to the data selection criteria, a total of 19 studies were included in the systematic literature review. The study selection process is shown in Figure 1.
The initial database search identified 2,771 records from international search engines (CINAHL, 58; Cochrane, 93; EMBASE, 1,509; PsycINFO, 101; and PubMed, 806) and Korean databases (DBpia, 25; KCI, 23; KISS, 18; Kmbase, 12; KoreaMed, 30; ScienceON, 39; and RISS, 57). In addition, nine records were added by searching the bibliographies of metaanalyses related to HLSIs, resulting in a total of 2,780 studies. After the removal of 609 duplicates, the remaining 2,171 records underwent title and abstract screening: 2,131 were excluded, and 40 papers were primarily selected. The full texts of these papers were retrieved, and 21 studies that did not meet the criteria for selection were excluded. Finally, 19 studies were identified for the final quality evaluation, and 13 studies were included in the meta-analysis.
2. Study CharacteristicsOf the 19 studies, 17 were RCTs and the other two were non-RCTs (Table 1). The studies included in the review involved 1,446 participants distributed in the United States [28-35], Australia [36], Spain [37,38], the Netherlands [39,40], Canada [41], Italy [42], Singapore [43], Hong Kong [44,45], and Taiwan [46] and the studies were performed between 2000 and 2019. The minimum sample size was 19 and the maximum was 266, and participants' mean age ranged from 9 to 29 years old. The cancer types included various childhood cancers, including acute lymphoblastic leukemia and brain tumors. The interventions consisted of exercise programs [30,31,35-39], educational programs [28,32-34,41,42,47], and combined exercise and educational programs [29,40,43-45]. Twelve interventions (63.2%) were conducted individually and seven (36.8%) were in group settings. The duration of the interventions varied from 1 week to 9 months, and five studies (26.3%) had interventions lasting longer than 6 months. In 17 out of 19 studies, one or more multi-modal interventions, such as exercise with counseling or education, and health interventions focusing on resilience, were applied. Physical interventions also combined two or more exercise types, including aerobic and anaerobic training. Several interventions were conducted using a mobile app, text messaging, or the phone instead of face-to-face interactions. The outcomes of the interventions mainly dealt with physical and psychosocial function and were measured with various tools and methods.
3. Study Quality
Figure 2 shows the results of the risk assessment of bias across all studies. Of the 17 RCTs included in this review, 10 (58.8%) fulfilled low risk of bias ratings across the five categories. Overall, no study had a high risk of bias and the results of all identifiable studies were included in the analysis to reduce the selection bias.
The two non-RCT studies included in this review were rated as having a high risk of bias. The study by Poggi et al. [42] had high bias in the measurement of intervention outcomes, and the study by Blaauwbroek et al. [39] had very high (critical) subject selection bias and a moderate bias due to deviation from the intended intervention.
4. Estimation of Effect SizeOut of the 19 studies screened for quality assessment, 13 (884 subjects, 12 RCTs and 1 non-RCT) were included in the meta-analysis, which measured this study's primary (HRQoL) and secondary outcomes (physical activity, fatigue, exercise capacity-VO2 peak, upper body, body mass index [BMI], and self-efficacy) (Figure 3). The included outcomes were selected only when three or more studies shared common measurement variables. Overall, all outcomes showed moderate effects with statistical significance (Z=3.94, d=-0.54, p<.001), yet a substantial amount of heterogeneity existed among the variables (I2=90%).
1) Tier I according to the study objectives: health-related quality of lifeHR-QoL, which was the study's primary outcome (n=8), showed a moderate effect size with statistical significance (d=-0.73, 95% CI=-1.25 to -0.21, p=.006), but high heterogeneity (I2=89%). To clarify the cause of heterogeneity, subgroup analyses were conducted (Figure 4) based on intervention type, intervention period, and group or individual approach. In these subgroup analyses, four studies showed significant effects (d=-0.46, 95% CI=-0.84 to -0.07, p=.02) when interventions were administered using a mixed approach (exercise and education), for not less than 6 months (n=4) (d=-0.72, 95% CI=-1.15 to -0.29, p=.001), and as a group approach (n=4) (d=-0.46, 95% CI=-0.85 to -0.06, p=.02), and there was a notable decrease in estimated heterogeneity in each analysis (I2 range: 68%-73%).
2) Tier II according to the study objectives: physical and psychosocial outcomesAmong the secondary outcomes, self-efficacy (n=5) showed a moderate effect (d=-0.74, 95% CI=-1.16 to -0.33, p=.0005), although measurable heterogeneity remained (I2=76%). Another subgroup analysis was performed based on identical standards (Supplement 1), showing small to moderate effect sizes and statistical significance when interventions were provided with health education only (n=2) (d=-0.55, 95% CI=-0.92 to -0.18, p=.003), for less than 6 months (n=3) (d=-0.40, 95% CI=-0.69 to -0.11, p=.006), and individually (n=2) (d=-0.55, 95% CI=-0.92 to -0.18, p=.003). The results of each analysis were found to be homogeneous (I2=0%).
The remaining secondary outcomes in this meta-analysis (physical activity, fatigue, exercise capacity–VO2, exercise capacity-upper body, and BMI) revealed no statistical significance (p-value range: .13 to .94) and the estimated heterogeneity between the included studies was high (I2 range: 87%-95%). Each outcome was subjected to subgroup analyses to explain potential causes of the lack of significant results, but the results remained unchanged both for effect size and heterogeneity. To assess publication bias, funnel plots were generated for all outcome variables, showing a somewhat asymmetrical distribution (Figure 3), which suggested that some bias may have existed.
DISCUSSION1. Study Characteristics and Study QualityIn this section, the characteristics and quality evaluation of the papers presented in Table 1 and Figure 2 are discussed.
According to previous review papers published since 2016 related to healthy lifestyles among CACS, adopting a healthy lifestyle plays a pivotal role in improving their HR-QoL [5,8,11,13]. The effects and associations of HLSIs for CACS have been reported, focusing on variables related to physical, psychological, social, and school adjustment [5,11-13,28,29,42,44, 46,47]. However, those meta-analyses have been conducted to identify the effects of interventions that dealt with only partial aspects of HLSIs. Based on the health concept presented by the WHO [14], no meta-study of the effects of HLSIs on CACS has been conducted from a holistic perspective, including physical, psychological, and spiritual aspects. This study aimed to identify the effects of interventions promoting a healthy lifestyle for CACS more comprehensively. The high heterogeneity of the results can be seen as reflecting the purpose of this study; simultaneously, however, these results paradoxically show that comprehensive intervention development is needed to promote a healthy lifestyle.
Three systematic reviews and meta-analyses [9-11] on interventions aiming to induce changes in health behavior published since 2016 found that regular exercise is a post-treatment recommendation because CACS have limitations in physical and fitness activity due to the sequelae of their condition. Thus, exercise is suggested as an important factor that improves health and well-being [10]. Furthermore, exercise has positive effects on their HR-QoL [11].
The remaining 12 studies described interventions that mixed exercise with psychosocial care or interventions that promoted the healthy psychosocial adaptation of CACS. Nonetheless, considering that humans' mental and spiritual attributes are closely related to their physical and psychological aspects, interventions for the spiritual well-being of CACS need to be further developed [12,13].
Four studies [30,31,36,38] that applied web or mobile-based interventions were published. Although distance-delivered interventions did not reach the conventional level of statistical significance for improving CACS' health outcomes, they were reported to reduce the risk of comorbidities and to have somatic and psychological benefits [9]. In terms of statistical significance, various results have been reported, yet these results suggest the need for further studies with a larger number of CACS. It should also be kept in mind that digital interventions such as eHealth and mHealth may have the potential to overcome future barriers associated with the anticipated need for physical distancing in the future due to factors such as the pandemic era, distance from trained providers, and competing life demands (e.g., school, physical condition, and family) [22,47]. Distance-based interventions also serve as an appealing tool for addressing the complex needs of CACS and connecting with them [48].
Interestingly, 15 [28-42] out of the 19 studies related to HLSIs were conducted in Western cultures. The other four studies were performed in Taiwan [46], Hong Kong [44,45], and Singapore [43]. Similar research trends were also identified in previous meta-analyses. Among the 13 systematic reviews or meta-analyses related to HLSIs in CACS from 2016 to 2020, 11 were conducted in Western cultures [4-10,12,13,20,22], and only two were performed in Asia—China [11] and Hong Kong [49]. More systematic attention and research are required to promote a healthy lifestyle for CACS in the Asian region.
Regarding the quality of the 17 RCTs, 10 studies showed a low risk of bias, indicating that their findings can be considered to be reliable, but the remaining seven studies presented "some concern". Furthermore, there were only two non-RCTs based on psychological interventions and one with a pilot study on exercise counseling; thus, it is difficult to make evidence-based suggestions. For this reason, it will be necessary to consider more stringent blinding processes for participant assignment with RCT designs in future research.
Additionally, in nine out of the 19 studies (Table 1) [29,32,33,35,43-47], theoretical models of interventions and outcome variables for promoting a healthy lifestyle (social cognitive theory, self-efficacy theory, learning theory, the precede-proceed model, and the health belief model) were presented. In the remaining 10 studies, a theoretical framework was not presented. Presenting the rationale for the intervention in an experimental study is presented can strengthen the scientific validity of the effect of the developed intervention. Future studies should revalidate the applied theory by identifying the significance of the effect measurement variables.
The WHO regards trial registration as the publication of an internationally agreed-upon set of information about the design, conduct, and administration of clinical trials. The trial registration status of the 17 RCT and 2 non-RCT papers included in this study was checked, and nine RCT studies [29,32,34-38,40,41] included the clinical trial registration number. In future studies, it will be necessary to consider the selection of papers that comply with the International Clinical Trials Registry. This may lead to improvements in the quality of clinical trials by making it possible to identify potential problems (such as problematic randomization methods) early in the research process [50].
2. Effect Size of Outcome VariablesThe primary outcome of this meta-analysis is that HR-QoL came close to having a large effect and was statistically significant, but it also had high heterogeneity. A meta-analysis [11] of the effects of exercise interventions on HR-QoL for CACS reported substantial variation according to exercise intensity and duration. The 13 studies included in this metaanalysis could also be classified according to the characteristics of intervention type (single or mixed), format (individual or group), and period (>6 months or <6 months). In a subgroup analysis of these three characteristics, the heterogeneity of effects on HR-QoL was lower for mixed interventions, interventions lasting >6 months, and group interventions. Assessing HR-QoL outcomes can provide prognostic and predictive information for clinicians, helping them understand the experiences of CACS and enabling them to develop effective lifestyle interventions [11]. When trying to improve the HR-QoL of CACS through HLSIs, the intervention type and duration are important factors to consider in program development.
As secondary outcomes of the effect size of HLSIs, the physical measurement variables available for meta-analysis were physical activity, fatigue, exercise capacity (VO2 peak, upper body), and BMI, but the effect sizes were not significant. Similarly, in the meta-analysis conducted by Zhi et al. [11], which investigated the effects of exercise interventions on adolescent and young adult cancer survivors, the overall effects related to physical outcomes were not significant. In contrast, statistically significant effects were reported in a meta-analysis of observational studies on physical activity and fitness [10] and in a meta-analysis regarding distance-delivered physical activity interventions for childhood cancer survivors [9]. A reason for the variation in significance of the exercise intervention effects from study to study seems to be that the types of interventions and the methods of measuring effects were particularly diverse. Moreover, the intensity of exercise intervention programs varied from light physical activity, such as walking, to vigorous physical activity such as aerobic exercise [11]. These variations led to limitations in identifying common exercise intervention designs and measurement methods.
Self-efficacy, which was identified as a psychosocial outcome variable in this study, showed a large effect size and was statistically significant. In the subgroup analysis, the heterogeneity was at 0% in single interventions, interventions lasting less than 6 months, and individual interventions, which were common features of the studies by Casillas et al. [28] and Wu et al. [46]. CACS face distinct barriers when navigating the healthcare system compared to adult cancer survivors. Mobile messaging technology is an appropriate way to reach out to CACS in order to disseminate health information. Therefore, they need age-appropriate educational interventions to optimize their long-term follow-up care [28]. The intervention characteristics, including intervention type (single or mixed), format (individual or group), and duration (>6 months or <6 months) that were found to affect heterogeneity in this study, are factors that should be considered in future intervention studies aiming to enhance the self-efficacy of CACS.
Regarding publication bias, which was analyzed using funnel plots, some studies included in the analysis fell outside the triangle, indicating that there may have existed some potential publication bias. This implies that it is necessary to confirm the effectiveness of HLSIs through future studies. Among the 19 studies included in the quality evaluation, the results of five were excluded from the meta-analysis. In these five studies [14,33,35,36,42,43], various variables (e.g., social skills, motivation, anxiety, and health locus of control) were used as intervention effects, but due to the small number of studies, a meta-analysis was not appropriate for evaluating those effects. In addition, various interventions for health promotion in CACS have been developed, but few studies have measured the degree of healthy lifestyle performance as an outcome variable. Based on Nola Pender's health promotion model, a healthy lifestyle measurement tool that measures adolescent health-promoting behaviors more comprehensively has been validated in several cultures [14]. This tool can be applied to future studies to verify the effectiveness of HLSIs for CACS.
3. Implications and LimitationsThis study identified that most of the interventions regarding HLSIs were exercise interventions with measured variables focused on physical aspects, and the more comprehensive effects of HLSIs for CACS have not yet been actively studied. This finding suggests the need for research on holistic health management.
Recently published meta-studies on HLSIs have not applied the holistic health concept to CACS, confirming the further need to develop HLSIs for holistic health management. Only a small number of intervention studies have been published that focus on psychosocial and spiritual care interventions for CACS and school adaptation, which also shows the need for vitalizing these research interventions.
Among the HLSIs' effect measurement variables, HR-QoL was found to be the most frequent outcome, which implied that the HR-QoL management of CACS is an important medical topic of interest. However, as the enhancement of HLSIs has been rarely measured, it is suggested that HLSIs should be reflected in effect size measurements along with HR-QoL in future studies. In addition, clinical trial enrollment among CACS is a topic that may have implications for the findings.
Based on the WHO's concept of health and a healthy lifestyle, this study focused on confirming the effects of HLSI studies from a holistic perspective. Therefore, all pediatric cancer types were included. Rather than using only RCT studies, two non-RCT studies were included in the quality evaluation and one in the meta-analysis. Therefore, this study had a limited ability to clarify the impact of randomization in research design. In addition, relatively few intervention studies [28,32,35,42,43,47] focusing on psychosocial and spiritual care interventions and school adaptations of CACS were published, which limited the estimation of the effect size. A metastudy on psychological adaptation of CACS with brain tumors [12] also reported a high risk of psychological adjustment difficulties, suggesting the future need for intervention development. The subgroup analysis results of this study have limitations in generalizability due to their small sample sizes. Unlike adult cancer, childhood cancer has a variety of occurrence times and types. Therefore, future research needs to identify the effects of HLSIs on HR-QoL according to cancer type and the child's developmental age.
CONCLUSIONHealthy lifestyle management is exceptionally important for improving the HR-QoL of CACS, and it is an important issue for healthcare providers who care for them. This meta-analysis is the first attempt to determine the effect size of HLSIs based on the holistic health concept. HLSIs were provided in the physical and psychosocial domains. Most of the interventions targeting the physical domain involved physical activities, including exercise, while interventions addressing the psychosocial domain provided education on cognitive factors. The outcomes were varied, although the interventions commonly focused on HR-QoL and self-efficacy. In addition, there were limitations in objectively examining the intervention effects due to differences in patients' severity and conditions.
Due to the lack of statistical significance in their effects, the inclusion of an exercise intervention and the duration and type of intervention (single/mixed, individual/group) can be considered as factors affecting the heterogeneity of HLSIs' effects on HR-QoL and self-efficacy. Nevertheless, it was found that these physical and psychosocial interventions provided positive results for patients' HR-QoL and self-efficacy. Until recently, HLSIs studies for CACS dealt with only single physical or psychosocial aspects. This study suggests that the degree of improvement in healthy lifestyles and HR-QoL should be measured using a holistic approach to promote a healthy lifestyle. In addition, since active participation is an important factor in HLSIs, participants' conditions inevitably affect the outcomes of the interventions. Therefore, it is also necessary to develop and apply programs that are not significantly affected by patients' conditions in daily life.
Supplementary materialSupplement 1.Subgroup Analyses of Health-related Quality of Life and Self-Efficacy NotesAuthors' contribution
Conceptualization: all authors; Data collection, Formal analysis: all authors; Writing-original draft: all authors; Writing-review and editing: all authors; Final approval of published version: all authors.
Conflict of interest
No existing or potential conflict of interest relevant to this article was reported.
REFERENCES1. Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al.; IICC-3 Contributors. International incidence of childhood cancer, 2001-10: a population-based registry study. The Lancet. Oncology. 2017;18(6):719-731. https://doi.org/10.1016/s1470-2045(17)30186-9
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. https://doi.org/10.1016/s0140-6736(17)33326-3
3. Huang IC, Brinkman TM, Kenzik K, Gurney JG, Ness KK, Lanctot J, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. Journal of Clinical Oncology. 2013;31(33):4242-4251. https://doi.org/10.1200/jco.2012.47.8867
4. Zabih V, Kahane A, O'Neill NE, Ivers N, Nathan PC. Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: a systematic review. Journal of Cancer Survivorship. 2019;13(5):713-729. https://doi.org/10.1007/s11764-019-00790-w
5. Skiba MB, McElfresh JJ, Howe CL, Crane TE, Kopp LM, Jacobs ET, et al. Dietary interventions for adult survivors of adolescent and young adult cancers: a systematic review and narrative synthesis. Journal of Adolescent and Young Adult Oncology. 2020;9(3):315-327. https://doi.org/10.1089/jayao.2019.0105
6. Tjon-A-Joe S, Pannekoek S, Kampman E, Hoedjes M. Adherence to diet and body weight recommendations among cancer survivors after completion of initial cancer treatment: a systematic review of the literature. Nutrition and Cancer. 2019;71(3):367-374. https://doi.org/10.1080/01635581.2018.1540713
7. Morales JS, Valenzuela PL, Rincón-Castanedo C, Santos-Lozano A, Fiuza-Luces C, Lucia A. Is health status impaired in childhood cancer survivors? A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology. 2019;142:94-118. https://doi.org/10.1016/j.critrevonc.2019.07.008
8. Pugh G, Gravestock HL, Hough RE, King WM, Wardle J, Fisher A. Health behavior change interventions for teenage and young adult cancer survivors: a systematic review. Journal of Adolescent and Young Adult Oncology. 2016;5(2):91-105. https://doi.org/10.1089/jayao.2015.0042
9. Mizrahi D, Wakefield CE, Fardell JE, Quinn VF, Lim Q, Clifford BK, et al. Distance-delivered physical activity interventions for childhood cancer survivors: a systematic review and meta-analysis. Critical Reviews in Oncology/Hematology. 2017;118:27-41. https://doi.org/10.1016/j.critrevonc.2017.08.008
10. Antwi GO, Jayawardene W, Lohrmann DK, Mueller EL. Physical activity and fitness among pediatric cancer survivors: a meta-analysis of observational studies. Supportive Care in Cancer. 2019;27(9):3183-3194. https://doi.org/10.1007/s00520-019-04788-z
11. Zhi X, Xie M, Zeng Y, Liu JE, Cheng ASK. Effects of exercise intervention on quality of life in adolescent and young adult cancer patients and survivors: a meta-analysis. Integrative Cancer Therapies. 2019;18:1534735419895590. https://doi.org/10.1177/1534735419895590
12. Sharkey CM, Espeleta HC, Traino KA, Roberts CM, Perez MN, Bakula DM, et al. Psychological adjustment outcomes among pediatric brain tumor survivors: a meta-analysis. Pediatric Blood & Cancer. 2020;67(10):e28644. https://doi.org/10.1002/pbc.28644
13. Di Giuseppe G, Thacker N, Schechter T, Pole JD. Anxiety, depression, and mental health-related quality of life in survivors of pediatric allogeneic hematopoietic stem cell transplantation: a systematic review. Bone Marrow Transplantation. 2020;55(7):1240-1254. https://doi.org/10.1038/s41409-020-0782-z
14. Gaete J, Olivares E, Godoy MI, Cárcamo M, Montero-Marín J, Hendricks C, et al. Adolescent lifestyle profile-revised 2: validity and reliability among adolescents in Chile. Jornal de Pediatria. 2021;97(1):52-60. https://doi.org/10.1016/j.jped.2019.11.005
15. U.S. Department of Health and Human Services. Healthy people 2020: adolescent health [Internet]. U.S. Department of Health and Human Services; 2020 [cited 2021 March 28]. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/Adolescent-Health
16. Zhang FF, Hudson MM, Huang IC, Bhakta N, Ness KK, Brinkman TM, et al. Lifestyle factors and health-related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2018;124(19):3918-3923. https://doi.org/10.1002/cncr.31647
17. Warner EL, Nam GE, Zhang Y, McFadden M, Wright J, Spraker-Perlman H, et al. Health behaviors, quality of life, and psychosocial health among survivors of adolescent and young adult cancers. Journal of Cancer Survivorship. 2016;10(2):280-290. https://doi.org/10.1007/s11764-015-0474-7
18. Ness KK, Gurney JG, Zeltzer LK, Leisenring W, Mulrooney DA, Nathan PC, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Archives of Physical Medicine and Rehabilitation. 2008;89(1):128-136. https://doi.org/10.1016/j.apmr.2007.08.123
19. Casillas J, Goyal A, Bryman J, Alquaddoomi F, Ganz PA, Lidington E, et al. Development of a text messaging system to improve receipt of survivorship care in adolescent and young adult survivors of childhood cancer. Journal of Cancer Survivorship. 2017;11(4):505-516. https://doi.org/10.1007/s11764-017-0609-0
20. Marjerrison S, Hendershot E, Empringham B, Nathan PC. Smoking, binge drinking, and drug use among childhood cancer survivors: a meta-analysis. Pediatric Blood & Cancer. 2016;63(7):1254-1263. https://doi.org/10.1002/pbc.25943
21. Ramsey WA, Heidelberg RE, Gilbert AM, Heneghan MB, Badawy SM, Alberts NM. eHealth and mHealth interventions in pediatric cancer: a systematic review of interventions across the cancer continuum. Psychooncology. 2020;29(1):17-37. https://doi.org/10.1002/pon.5280
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
23. Higgins JPT, Savović J, Page MJ, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [Internet]. RoB2 Development Group; 2019 [cited 2022 February 10]. Available from: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2
24. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
25. Cochrane Collaboration. Review manager (RevMan) Version 5.3 [software]. 2014 [cited 2021 March 28]. Available from: https://revman.cochrane.org/info
26. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. p. 24.
27. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 [Internet]. The Cochrane Collaboration; 2022 [cited 2022 February 8]. Available from: www.training.cochrane.org/handbook.
28. Casillas JN, Schwartz LF, Crespi CM, Ganz PA, Kahn KL, Stuber ML, et al. The use of mobile technology and peer navigation to promote adolescent and young adult(AYA) cancer survivorship care: results of a randomized controlled trial. Journal of Cancer Survivorship. 2019;13(4):580-592. https://doi.org/10.1007/s11764-019-00777-7
29. Devine KA, Viola A, Levonyan-Radloff K, Mackowski N, Bozzini B, Chandler A, et al. Feasibility of FitSurvivor: a technology-enhanced group-based fitness intervention for adolescent and young adult survivors of childhood cancer. Pediatric Blood & Cancer. 2020;67(9):e28530. https://doi.org/10.1002/pbc.28530
30. Howell CR, Krull KR, Partin RE, Kadan-Lottick NS, Robison LL, Hudson MM, et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatric Blood & Cancer. 2018;65(8):e27216. https://doi.org/10.1002/pbc.27216
31. Huang JS, Dillon L, Terrones L, Schubert L, Roberts W, Finklestein J, et al. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatric Blood & Cancer. 2014;61(5):894-900. https://doi.org/10.1002/pbc.24937
32. Hudson MM, Tyc VL, Srivastava DK, Gattuso J, Quargnenti A, Crom DB, et al. Multi-component behavioral intervention to promote health protective behaviors in childhood cancer survivors: the protect study. Medical and Pediatric Oncology. 2002;39(1):2-1. discussion 2. https://doi.org/10.1002/mpo.10071
33. Kunin-Batson A, Steele J, Mertens A, Neglia JP. A randomized controlled pilot trial of a web-based resource to improve cancer knowledge in adolescent and young adult survivors of childhood cancer. Psychooncology. 2016;25(11):1308-1316. https://doi.org/10.1002/pon.3956
34. Mays D, Black JD, Mosher RB, Heinly A, Shad AT, Tercyak KP. Efficacy of the survivor health and resilience education (SHARE) program to improve bone health behaviors among adolescent survivors of childhood cancer. Annals of Behavioral Medicine. 2011;42(1):91-98. https://doi.org/10.1007/s12160-011-9261-5
35. Mendoza JA, Baker KS, Moreno MA, Whitlock K, Abbey-Lambertz M, Waite A, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: a pilot study. Pediatric Blood & Cancer. 2017;64(12):e26660. https://doi.org/10.1002/pbc.26660
36. Atkinson M, Murnane A, Goddard T, Pendergrast C, Rogers P, Manudhane R, et al. A randomized controlled trial of a structured exercise intervention after the completion of acute cancer treatment in adolescents and young adults. Pediatric Blood & Cancer. 2021;68(1):e28751. https://doi.org/10.1002/pbc.28751
37. Fiuza-Luces C, Padilla JR, Soares-Miranda L, Santana-Sosa E, Quiroga JV, Santos-Lozano A, et al. Exercise intervention in pediatric patients with solid tumors: the physical activity in pediatric cancer trial. Medicine and Science in Sports and Exercise. 2017;49(2):223-230. https://doi.org/10.1249/mss.0000000000001094
38. Manchola-González JD, Bagur-Calafat C, Girabent-Farrés M, Serra-Grima JR, Pérez RÁ, Garnacho-Castaño MV, et al. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: results of a randomised clinical trial. Supportive Care in Cancer. 2020;28(7):3171-3178. https://doi.org/10.1007/s00520-019-05131-2
39. Blaauwbroek R, Bouma MJ, Tuinier W, Groenier KH, de Greef MH, Meyboom-de Jong B, et al. The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: a pilot study. Supportive Care in Cancer. 2009;17(8):1041-1048. https://doi.org/10.1007/s00520-008-0533-y
40. van Dijk-Lokkart EM, Braam KI, van Dulmen-den Broeder E, Kaspers GJ, Takken T, Grootenhuis MA, et al. Effects of a combined physical and psychosocial intervention program for childhood cancer patients on quality of life and psychosocial functioning: results of the QLIM randomized clinical trial. Psychooncology. 2016;25(7):815-822. https://doi.org/10.1002/pon.4016
41. Barrera M, Atenafu EG, Sung L, Bartels U, Schulte F, Chung J, et al. A randomized control intervention trial to improve social skills and quality of life in pediatric brain tumor survivors. Psychooncology. 2018;27(1):91-98. https://doi.org/10.1002/pon.4385
42. Poggi G, Liscio M, Pastore V, Adduci A, Galbiati S, Spreafico F, et al. Psychological intervention in young brain tumor survivors: the efficacy of the cognitive behavioural approach. Disability and Rehabilitation. 2009;31(13):1066-1073. https://doi.org/10.1080/09638280802509546
43. Chung OK, Li HC, Chiu SY, Ho KY, Lopez V. Sustainability of an integrated adventure-based training and health education program to enhance quality of life among Chinese childhood cancer survivors: a randomized controlled trial. Cancer Nursing. 2015;38(5):366-374. https://doi.org/10.1097/ncc.0000000000000211
44. Li HC, Chung OK, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psychooncology. 2013;22(11):2601-2610. https://doi.org/10.1002/pon.3326
45. Li WHC, Ho KY, Lam KKW, Lam HS, Chui SY, Chan GCF, et al. Adventure-based training to promote physical activity and reduce fatigue among childhood cancer survivors: a randomized controlled trial. International Journal of Nursing Studies. 2018;83:65-74. https://doi.org/10.1016/j.ijnurstu.2018.04.007
46. Wu LM, Chen CM, Hsu HT, Liu Y, Su HL. Tailored education enhances healthy behaviour self-efficacy in childhood cancer survivors: a randomised controlled study with a 4-month follow-up. European Journal of Cancer Care. 2019;28(4):e13063. https://doi.org/10.1111/ecc.13063
47. Berg CJ, Stratton E, Esiashvili N, Mertens A. Young adult cancer survivors' experience with cancer treatment and follow-up care and perceptions of barriers to engaging in recommended care. Journal of Cancer Education. 2016;31(3):430-442. https://doi.org/10.1007/s13187-015-0853-9
48. Devine KA, Viola AS, Coups EJ, Wu YP. Digital health interventions for adolescent and young adult cancer survivors. JCO Clinical Cancer Informatics. 2018;2:1-15. https://doi.org/10.1200/cci.17.00138
49. Poon LHJ, Yu CP, Peng L, Ewig CL, Zhang H, Li CK, et al. Clinical ascertainment of health outcomes in Asian survivors of childhood cancer: a systematic review. Journal of Cancer Survivorship. 2019;13(3):374-396. https://doi.org/10.1007/s11764-019-00759-9
50. World Health Organization. International clinical trials registry platform (ICTRP) [Internet]. World Health Organization; 2022 [cited 2022 March 20]. Available from: www.who.int/clinical-trials-registry-platform/.
Figure 1.PRISMA flowchart of database search results on healthy lifestyle interventions for childhood and adolescent cancer survivors. RCT, randomized controlled trials. 
Figure 2.Risk of bias graph of quality assessment. D1, randomization process; D2, deviations from the intended intervention; D3, missing outcome data; D4, measurement of the outcome; D5, selection of the reported result. 
Figure 3.Forest plots of effect size and 95% CI of meta-analysis on healthy lifestyle interventions for childhood and adolescent cancer survivors (17 studies). CI, confidence interval; IV, inverse variance; SD, standard deviation. 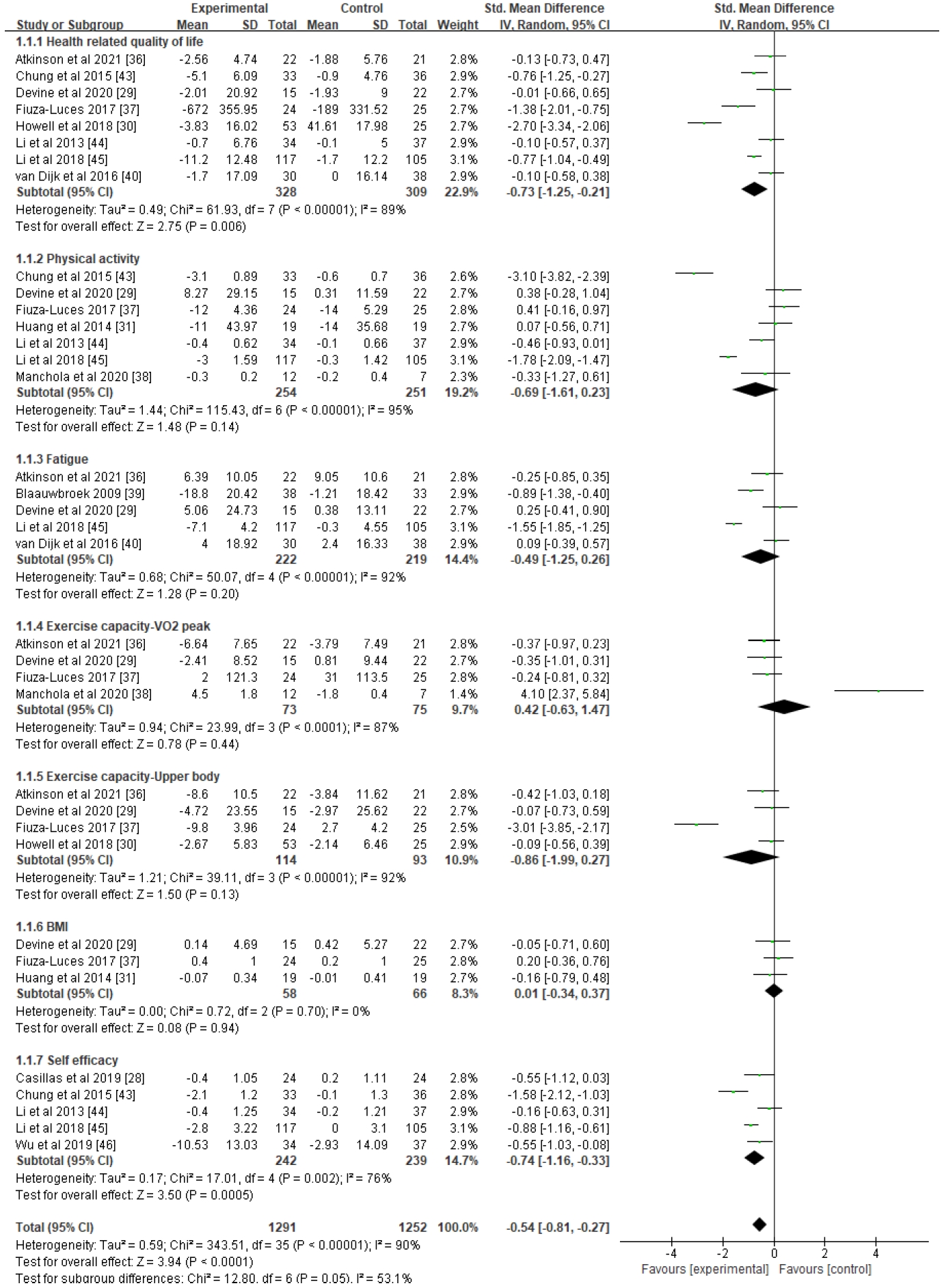
Figure 4.Funnel plots of effect sizes for seven outcomes of the meta-analysis on healthy lifestyle interventions for childhood and adolescent cancer survivors (17 studies). BMI, body mass index. 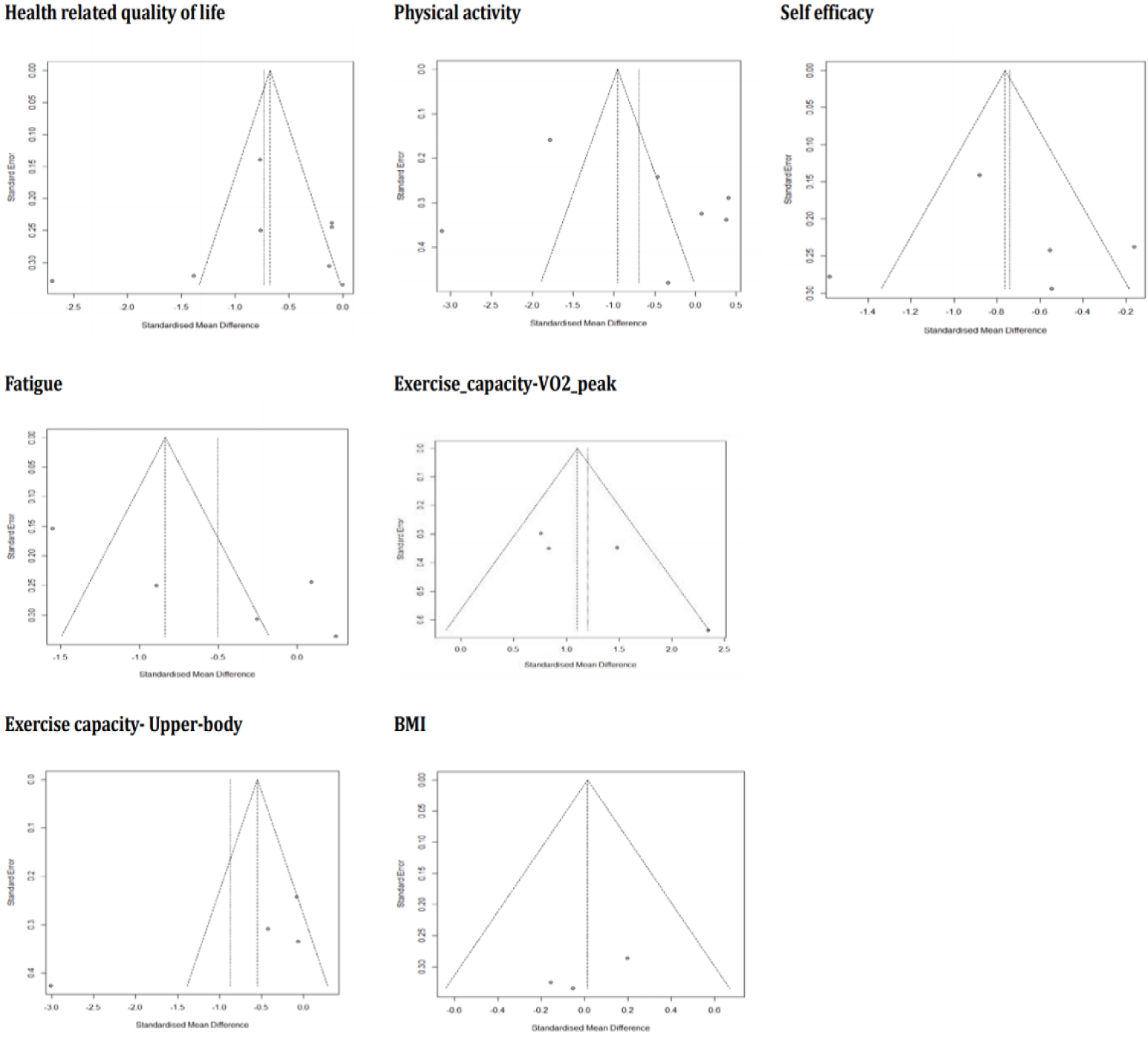
Table 1.Descriptive Summary of Included Studies on Healthy Lifestyle Interventions for Childhood and Adolescent Cancer Survivors
ALL, acute lymphoblastic leukemia; BCSC, Brinkerhoff's Computer Self-efficacy Scale; BMI, body mass index; BP, blood pressure; BREQ, Behavioral Regulation in Exercise Questionnaire; CBCL, Child Behavior Checklist; CDI, Children's Depression Inventory; CIS, Checklist Individual Strength; CTCAEV4, Common Terminology Criteria for Adverse Events version 4; CUHK-PARCY, Chinese University of Hong Kong: Physical Activity Rating for Children and Youth; D-KEFS, Delis-Kaplan Executive Function System; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; FEV1 , forced expiratory volume; FS-C, Fatigue Scale-child; GSLTPAQ, Godin-Shephard Leisure Time Physical Activity Questionnaire; HBSE, health behavioral self-efficacy; HDL, high density lipoprotein; HLC, health locus of control; HPL, health promotion lifestyle; IPAQ-SF, International Physical Activity Questionnaire-Short Form; LDL, low density lipoprotein; MVPA, moderate-vigorous physical activity; PACE, Patient-centered Assessment & Counseling for Exercise; PAES, Physical Activity Enjoyment Scale; PAQ-A, Physical Activity Questionnaire for Adolescents; PA-SE, Physical Activity Self-efficacy; PASCQ, Physical Activity Stages of Change Questionnaire; PedsQL, pediatric quality of life; RER, respiratory exchange ratio; RR peak, respiratory rate peak; SCAC, Survivorship Care Attitude Scale; SCKS, Survivorship Care Knowledge Scale; SCPSC, Survivorship Care Planning Self-efficacy Scale; SOC, Stage of Change Questionnaire; SPP, Self-perception Profile; SRSCK, Self-reported Survey of Cancer Knowledge; SSRS, Social Skills Rating System; STAI, State Trait Anxiety Inventory; TUDS, timed up and down stairs test; TUG, timed up and go test; VABS, Vineland Adaptive Behavior Scales (expanded form); VO2peak, peak oxygen consumption; WASI, Wechsler Abbreviated Scale of Intelligence; YAQ, Youth Adolescent Questionnaire. |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||